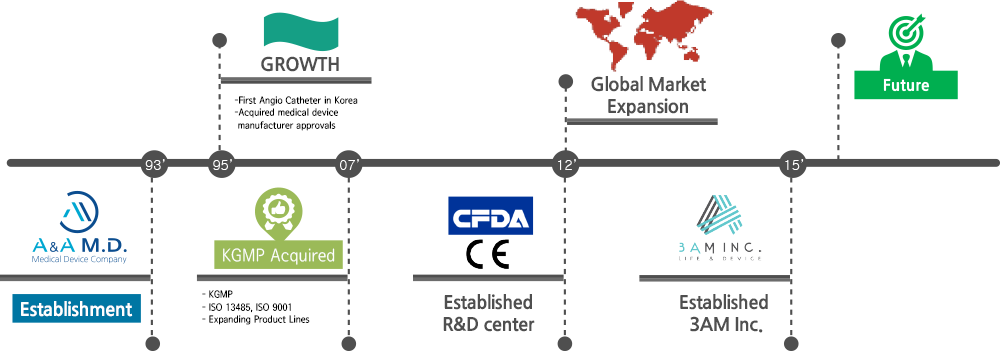
History of Challenges and Growth

10
Changed the 3AM LOGO
6
KGMP Certified : 3AM
![]()
10
Organized Medical Device Business Team
Construction on New 3AM Headquarter
8
Acquired Certificate of Manufacturer
5
Established a Subsidiary Company: 3AM Inc.
![]()
7
CFDA Certified: Tool Kit
![]()
11
CFDA Certified: Balloon Catheter
2
Established R&D center
![]()
4
Added Carina Catheter
![]()
10
Company Name Changed to A&A M.D.
12
Added PTCA Balloon Catheter
![]()
17
Acquired the Medical Instrument Manufacturing Item Certificate from the Ministry of Health and Welfare / Balloon Catheter / no 04-873
![]()

1
Change of the Representative
![]()

1.13
Enrolled as the member of the Korean Industrial Trade Association (KITA) (11240112)
4.15
Acquired the Medical Instrument Manufacturing Item Certificate from the Ministry of Health and Welfare / Guiding catheter / no.6
![]()

4.6
Acquired the Medical Instrument Manufacturing Item Certificate from the Korean Food & Drug Administration
Acquired the Manufacture Business Certificate from the Ministry of Health and Welfare ( no. 524)
![]()

10
Factory enrollment of the JungSung Technical Business (SeongNam Mayor)
12.7
Relocation of the current company to the Technopark at the YaTapDong, BunDang District in SeongNam City, Kyunggi Province
12.15
Manufactured the nation’s first “Angio Catheter” with the addition of the Hair Wire, Guide Wire, Yellow Sheath and PTC Needle items.
![]()

11.8
Established the JungSung Technical Business (129-14-98215)
I am text block. Click edit button to change this text. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.





